Resource Hub for Health Technology Assessment at IIPHG, Gujarat
Role of Hub :
In April 2018, IIPHG established a Regional Resource Centre for Health Technology Assessment (HTA-RRC) with support from the Department of Health Research (DHR), Government of India. Recognizing its continued contributions to evidence-based health policymaking, this centre was upgraded to a Resource Hub in April 2024 by the DHR, Ministry of Health and Family Welfare, Government of India.
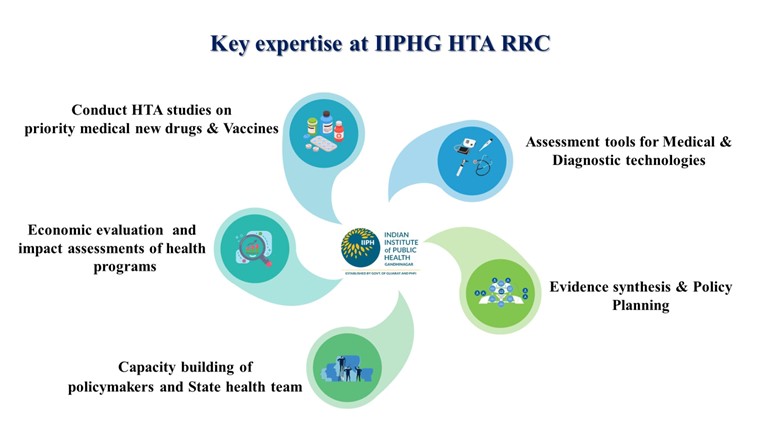
The Resource Hub operates with the vision of facilitating the development of safe, effective, and cost-efficient population-centric health policies. It is supported by a dedicated, multidisciplinary team of trained scientists specializing in health technology assessments and economic evaluations, catering to the Western Zone of India.
Strategic Collaborations and Governance in Gujarat
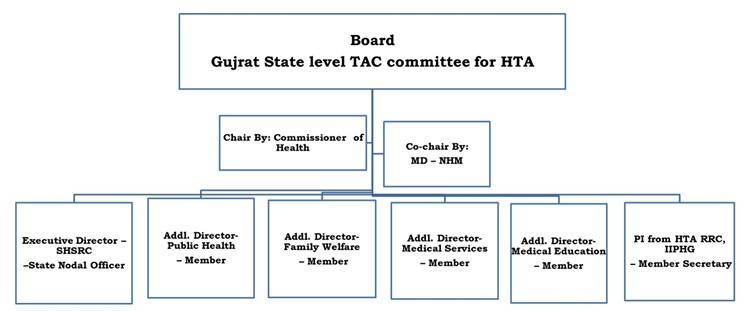
Resource Hub works in close collaboration with the Department of Health research (DHR) HTAIn division and the State Department of Health and Family Welfare (H&FW), Government of Gujarat. In recognition of its significant contributions to evidence-informed decision-making, the Gujarat Technical Advisory Group on HTA (TAG) was constituted in 2022. The committee is chaired by the Commissioner, H&FW, Government of Gujarat, and co-chaired by the Mission Director, National Health Mission, Gujarat. All four Additional Directors of H&FW serve as ex-officio members, ensuring interdepartmental coordination and strategic guidance. The Executive Director of the State Health Systems Resource Centre, Gujarat, has been nominated as the ex-officio State Nodal Officer for HTA in Gujarat. The Principal Investigator of the IIPHG HTA Resource Hub serves as the Member Secretary of the TAG, facilitating technical and operational coordination in Gujarat State.
Gujrat State level TAC Committee Members: